
How to Calculate the Formal Charge of CoCl2 Sciencing
The IUPAC name for the COCl 2 molecule is carbonyl dichloride. It is a toxic, colorless gas that emits a suffocating odor. It is more commonly known as phosgene. Phosgene is particularly important as a reagent in the polymer and plastic manufacturing industry.

Draw the Lewis structure for COCl2, including lone pairs.What is the
What is this molecule? COCl 2 is the chemical formula for carbonyl chloride, also known as phosgene. It is a colorless gas at room temperature with a pungent odor. Phosgene is a highly toxic compound that was historically used as a chemical warfare agent during World War I.

Cf4 molecular geometry studentxoler
3a. Drawing the structure of CoCl2 Table view List view 3a. Drawing the structure of CoCl2 COCI Lone pairs of electrons (central atom) Bonding groups (central atom) Total valence electrons VSEPR Molecular shape (central atom) Choose Choose. 3b. Evaluating the structure of CoCl2 Table view List view 3b.
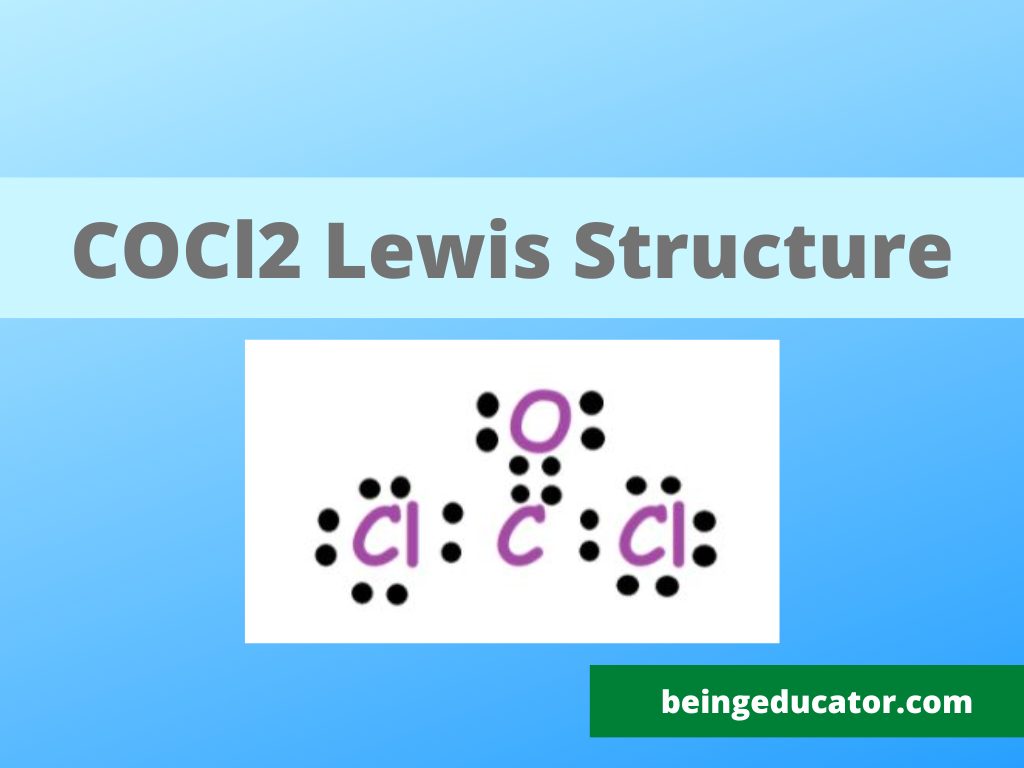
COCl2 Lewis Structure Hybridization Polarity Molecular Geometry
COCl2 (Phosgene) Molecular Geometry, Bond Angles (and Electron Geometry) Wayne Breslyn 726K subscribers Join Subscribe Subscribed 21K views 2 years ago An explanation of the molecular.

a Structural descriptions of an optimized free phosgene (COCl2
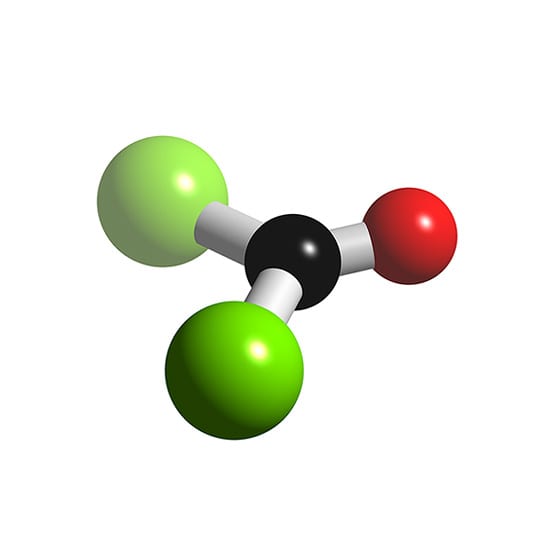
Structure and basic properties Phosgene is a planar molecule as predicted by VSEPR theory. The C=O distance is 1.18 Å, the C−Cl distance is 1.74 Å and the Cl−C−Cl angle is 111.8°. [9] Phosgene is a carbon oxohalide and it can be considered one of the simplest acyl chlorides, being formally derived from carbonic acid . Production
18+ Electron Geometry Vs Molecular Geometry Gif GM
Steps of drawing COCl2 lewis structure Step 1: Find the total valence electrons in COCl2 molecule. In order to find the total valence electrons in a COCl2 molecule, first of all you should know the valence electrons present in carbon atom, oxygen atom as well as chlorine atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
PPT The Shapes of Molecules PowerPoint Presentation, free download
Molecular Formula Cl2Co Synonyms 7646-79-9 Cobaltchloride Cobalt chloride (CoCl2) MFCD00010938 Cobalt (II) chloride, ultra dry View More. Molecular Weight 129.84 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Dates Create: 2005-03-26 Modify: 2023-12-30 Description Formerly approved as a food additive but now prohibited
COCl2 Phosgene
It is an inorganic compound that comprises Cobalt and Chlorine atoms. CoCl2 is a crystalline solid that is sky-blue in color. It is readily soluble in water, alcohol, and acetone. It occurs at different levels of hydration as dehydrates and hexahydrates. These versions of the salt are purple and pink, respectively.
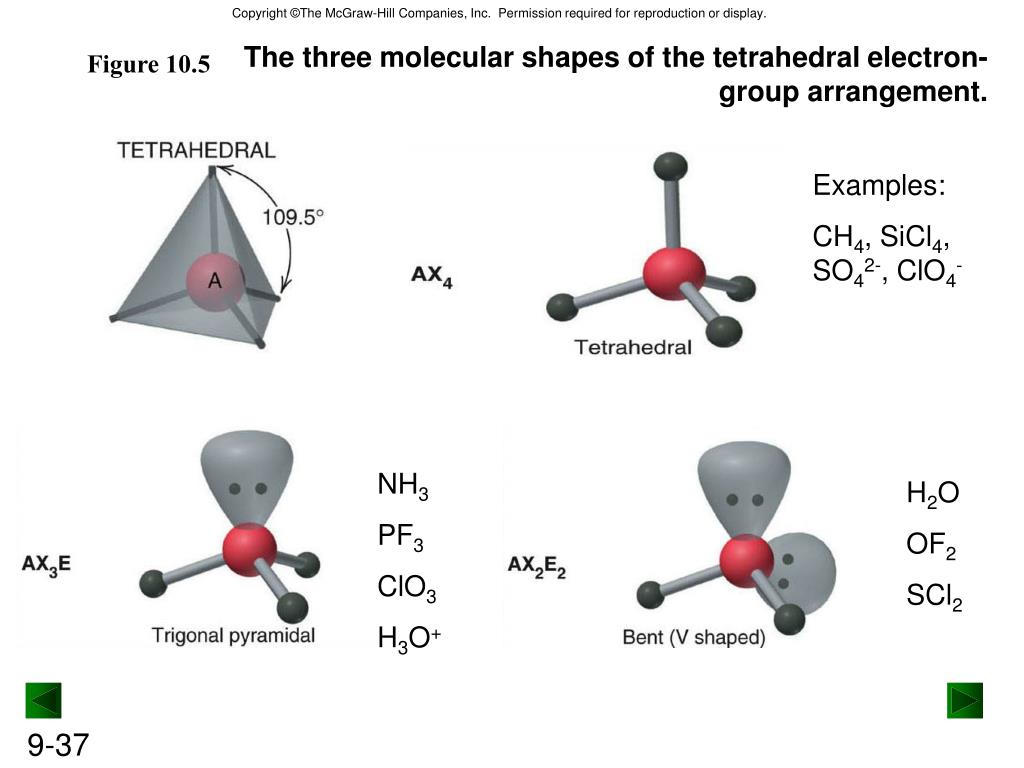
PPT Ch. 9 and 10 Chemical Bonding PowerPoint Presentation, free
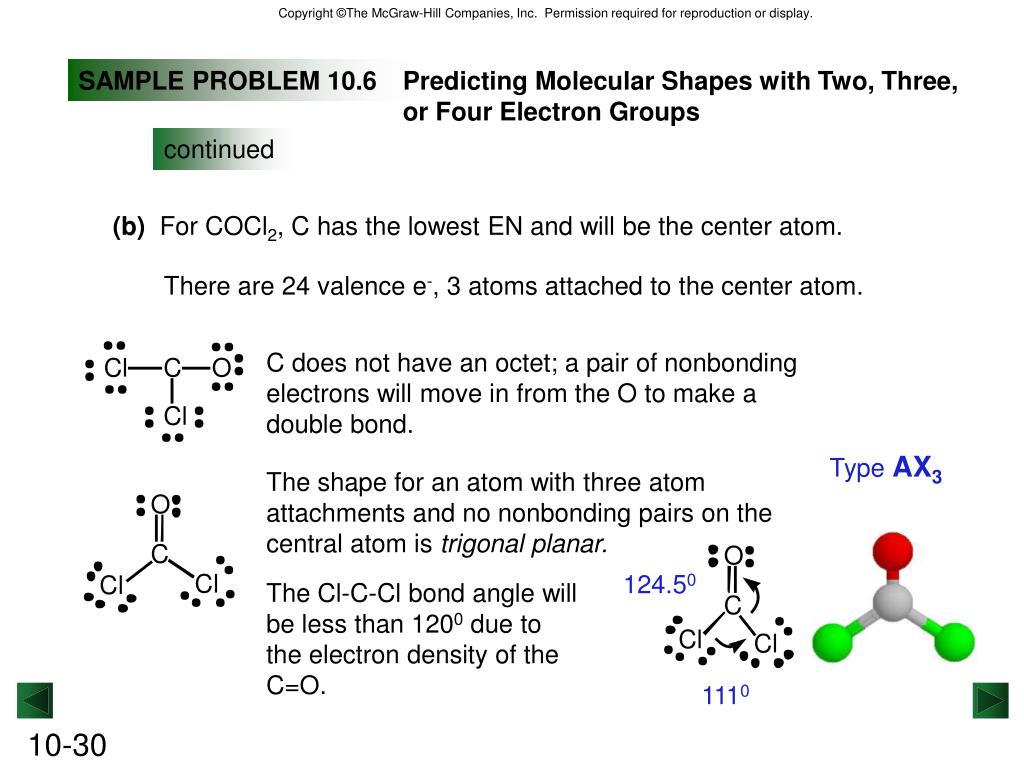
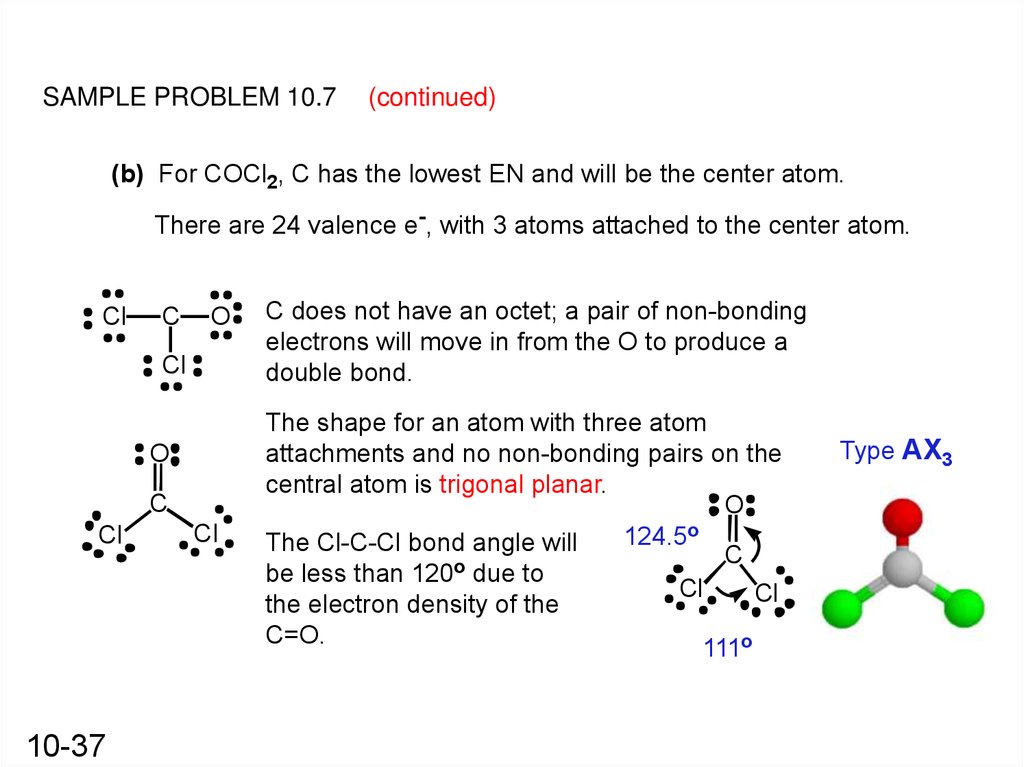
By Aparna Dev The Lewis structure of COCl2, also known as carbonyl chloride or phosgene, is a representation of its molecular structure using Lewis symbols and lines to show the bonding between atoms. In this structure, carbon is the central atom bonded to two oxygen atoms and one chlorine atom.

SOLVED Draw the Lewis structure for COCl2, including lone pairs.What
The crystal unit of the solid hexahydrate CoCl 2 •6 H 2O contains the neutral molecule trans - CoCl 2(H 2O) 4 and two molecules of water of crystallization. [8] This species dissolves readily in water and alcohol . The anhydrous salt is hygroscopic and the hexahydrate is deliquescent. [citation needed]

Draw the Lewis structure for COCl2, including lone pairsWhat is the
In the COCl 2 Lewis structure, there are two single bonds and one double bond around the carbon atom, with two chlorine atoms and one oxygen atom attached to it. Two chlorine atoms with single bonds have three lone pairs, and one oxygen atom with a single bond has two lone pairs. COCl2 Lewis Structure - How to Draw the Lewis Structure for COCl2
Seite nicht gefunden telekine fernsehproduktion
The molecular shape of COCl 2 is trigonal planar, or AX 3 using Valence Shell Electron Pair Repulsion (VSEPR) theory. Hence, the molecular geometry of COCl 2 only has 120 degree bond angles in the molecule. COCl 2 looks like this: How do you find the molecular geometry of COCl2?
Best Cocl2 Lewis Structure Molecular Geometry Pics GM
Molecular Formula CCl2O COCl2 Synonyms PHOSGENE Carbonyl dichloride Carbonic dichloride Carbonyl chloride Phosgen View More. Molecular Weight 98.91 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Dates Create: 2004-09-16 Modify: 2023-12-30 Description Phosgene is a colorless nonflammable gas that has the odor of freshly cut hay.

COCl2 (Phosgene) Molecular Geometry, Bond Angles (and Electron Geometry
COCl2 molecule consists of one C, one O, and Cl atoms. The total number of valence electrons = 4 + 6 + 7*2 = 10 + 14 = 24. Step 2: Now, we will have to find out the element which will take up the position of the central atom.

COCl2 Molecular Geometry, Bond Angles (Phosgene) YouTube
Molecular Formula Cl Co Average mass 129.839 Da Monoisotopic mass 128.870911 Da ChemSpider ID 22708 - Charge More details: Names Properties Searches Spectra Vendors Articles More Names and Synonyms Database ID (s) Validated by Experts, Validated by Users, Non-Validated, Removed by Users Cobalt (II) chloride [Wiki] 231-589-4 [EINECS] 7646-79-9 [RN]
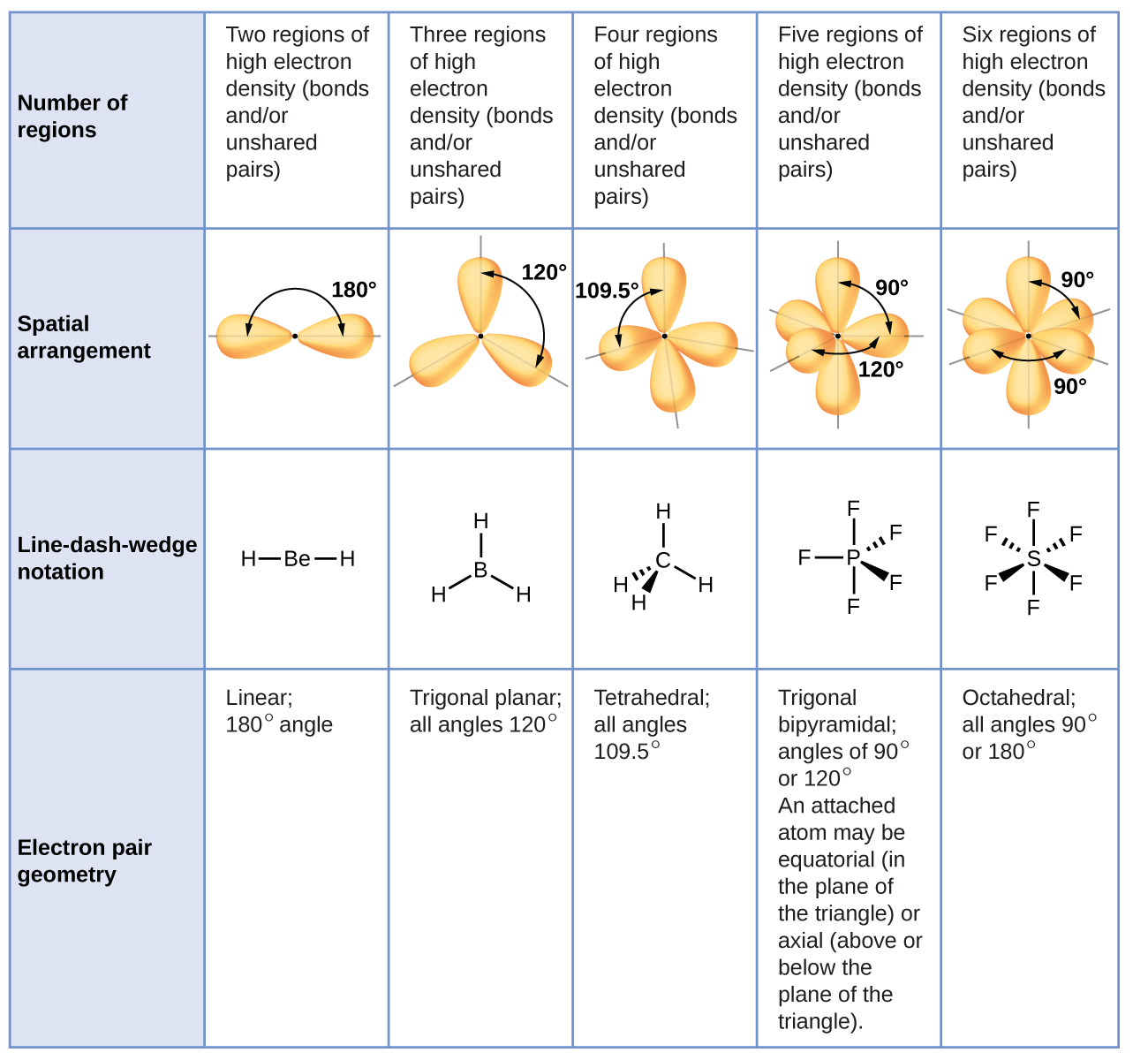
The Shapes of Molecules презентация онлайн
A step-by-step explanation of how to draw the COCl2 Lewis Dot Structure (Phosgene).For the COCl2 structure use the periodic table to find the total number of.