
Chemistry class 9 chapter 2 video 1 YouTube
In this chapter you are going to learn about atoms and molecules with the help of concepts, NCERT questions and extra Teachoo questions prepared by experts.. You will start learning l aws of chemical combination which includes -. Law of conservation of mass; Law of constant proportions; Secondly, you will learn about what an atom is and its properties which includes atomic mass.
chemistry class 9 chapter 3 solved exercise Pdf download?
In Text Questions Page No: 32 1. In a reaction, 5.3 g of sodium carbonate reacted with 6 g of ethanoic acid. The products were 2.2 g of carbon dioxide, 0.9 g water and 8.2 g of sodium ethanoate. Show that these observations are in agreement with the law of conservation of mass.

SOLUTION Class 9 full Chemistry notes solved exercises and notes Studypool
Questions related to Chapter 3 are frequently included in Class 9 Science examinations. This chapter is a crucial part of the curriculum, and students can expect to see questions about atoms, molecules, chemical equations, and related calculations in their exams. Class 9 science chapter 3 involves solving numerical problems related to.

Class 9 Chemistry Worksheet on Chapter 1 Matter in Our Surroundings Set 1
Get Access to Important Questions for CBSE Class 9 Science Chapter 3 Atoms and Molecules prepared by subject experts from the latest edition. Practicing this set of questions for Class 9 Science will help you understand every aspect of the chapter and help you score well in your exams. Learning App K-12 JEE NEET SAT PSAT TOEFL CAP CUET Teaching App

Adamjee Coaching Organic Chemistry MCQs Chemistry IX
Free PDF download of Extra Questions for CBSE Class 9 Science (Physics, Chemistry, and Biology) prepared by expert Science teachers at LearnCBSE.

CBSE Class 9 Science Chapter 3 Atoms And Molecules CBSE Study Group
Answer: The sum of the atomic masses of all the atoms in a molecule of the substance is expressed.in atomic mass unit. E.g., H 2 0 = 1 × 2 + 16 = 18 amu 8: How do atoms exist? Answer: Atoms exist in the form of atom, molecule or ions. 9: Give the atomicity of phosphorous and nitrogen. Answer: The atomicity of phosphorus is P 4 i.e., 4.

example 2.12 class 12 chemistry
Check CBSE Class 9 Science extra questions for Chapter 3 - Atoms and Molecules. All the questions and answers are important for exams to be held in 2020-2021. By Gurmeet Kaur.

Chemistry Class 9 Chapter 3 (3.1 ) First Half YouTube
NCERT Solutions for Class 9 Science Chapter 3 Atoms and Molecules (Hindi Medium) MORE QUESTIONS SOLVED. NCERT Solutions For Class 9 Science Chapter 3 Multiple Choice Questions. Choose the correct option: 1. The atomicity of K 2 Cr 2 O 7 is (a) 9 (b) 11 (c) 10 (d) 12 2. The formula for quick lime is (a) CaCl 2 (b) CaCO 3 (c) Ca(OH) 2 (d) CaO 3.

Important Questions For Class 9 Science Chapter 3 Atoms And Molecules Download PDF
Answer. (i) The exact number of atoms present in 12 gm of carbon-12 is called Avogadro constant. (ii) No of moles = Given Mass/Molar Mass = m/M. = 56/20 = 2.8. Q4. (i) Write the chemical formula of a compound using zinc ion and phosphate ion. (ii) Calculate the ratio by mass of atoms present in a molecule of carbon dioxide.
Chapter 3 Chemistry 9th Class Notes Matric Part 1 Notes
These CBSE Class 9 Science Chapter 3 important questions help students in learning the essential concepts of the chapter. We at BYJU'S provide these important questions so that students don't need to put extra effort in noting down important concepts.

Chemistry Chapter 4 Section 1 Review Answers TracehasRowe
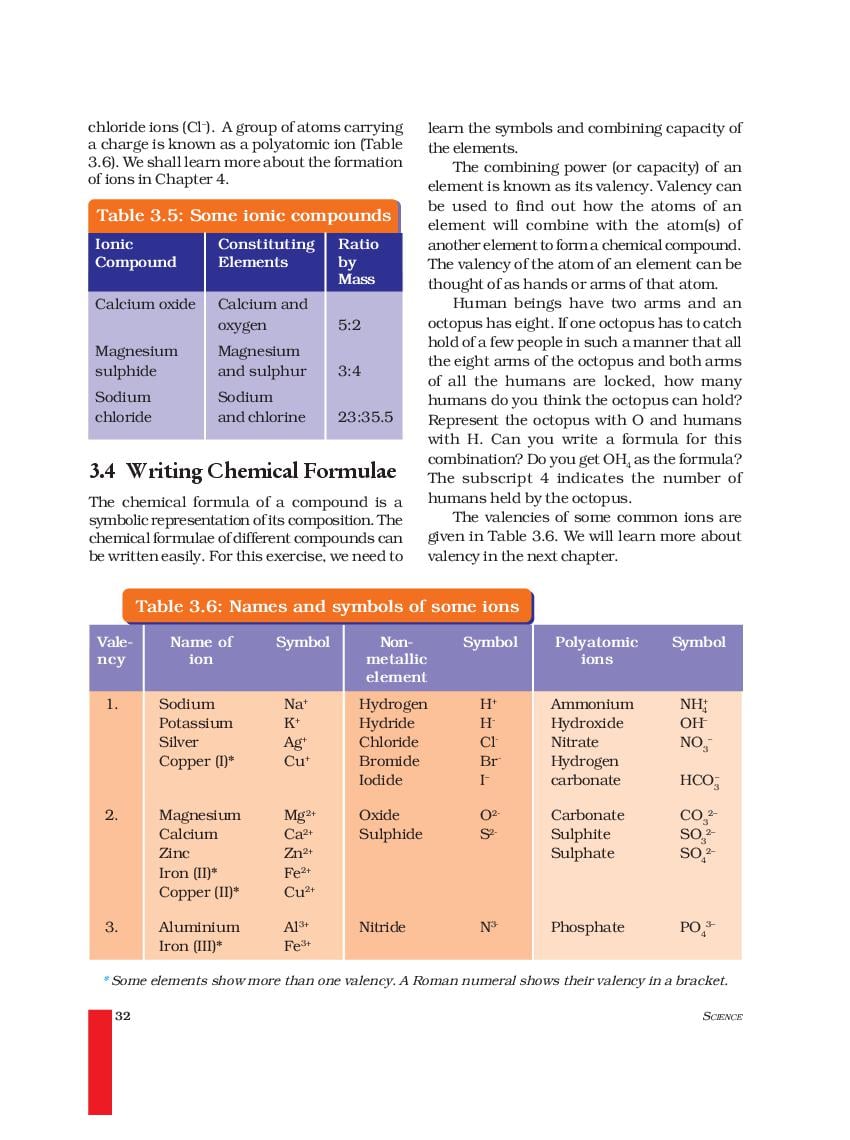
Question 1. Define atomic mass unit. Answer: Atomic mass unit of an element is one twelfth (1/12th) of the mass of one atom of carbon-12. Question 2. Write the valency of sulphur in H 2 S, SO 2 and SO 3. Answer: The valency of sulphur in H 2 S, SO 2 and SO 3 are 2, 4, and 6 respectively. Question 3. What is the difference between 2H and H 2?

Buy Chemistry Class 9 S.Chand Science Book Buy Old Book
In addition, the exemplar has objective questions, very short-answer types, short answer types and long-type questions for Class 9 Chapter 3. It is highly recommended for the students who wish to appear for competitive examinations. The NCERT Solutions Class 9 Science Chapter 3 has questions from the exemplar.

Chemistry class9 chapter 1 video 9 YouTube
1. Name the scientist who laid the foundation of chemical sciences. How ? Answer. Antoine Laurent Lavoisier, by establishing two important laws of chemical combination. Question. 2. Define law of conservation of mass. Answer. It states that, 'Mass is neither created nor destroyed in a chemical reaction.'

mixtures and solutions website
Best courses for you Crash course Starting from ₹ 1,111 Watch videos on Important Questions for CBSE Class 9 Science Atoms and Molecules 2023-24 Atoms and Molecules in One Shot (Full Chapter) | CBSE Class 9 Science Chapter 3 | Term 1 Preparation Vedantu 9&10 Subscribe Share 3.9K likes 141.6K Views

9Th Sindh Board Chemistry Text Book 11th Class Chemistry Book1 Educatedzone Ncert
Class 9 chemistry MCQs with answers are provided here for chapter 3 Atoms and Molecules. These MCQs are based on CBSE board curriculum and correspond to the most recent Class 9 chemistry syllabus.
NCERT Book Class 9 Science Chapter 3 Atoms And Molecules
You can learn the mole concept in detail in Atoms and Molecules Class 9. Exercise 3.5 total Solutions: 11 Questions (6 short question and 5 Long questions).. Chemistry Chapter 3 Class 9 NCERT answers use a point-by-point method to assist students to review the material before the test. When students work through the atoms and molecules class.